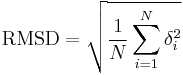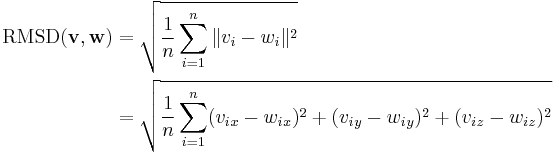Root-mean-square deviation (bioinformatics)
The root-mean-square deviation (RMSD) is the measure of the average distance between the atoms (usually the backbone atoms) of superimposed proteins. In the study of globular protein conformations, one customarily measures the similarity in three-dimensional structure by the RMSD of the Cα atomic coordinates after optimal rigid body superposition.
When a dynamical system fluctuates about some well-defined average position the RMSD from the average over time can be referred to as the RMSF or root-mean-square fluctuation. The size of this fluctuation can be measured, for example using Mössbauer spectroscopy or nuclear magnetic resonance, and can provide important physical information. The Lindemann index is a method of placing the RMSF in the context of the parameters of the system.
A widely used way to compare the structures of biomolecules or solid bodies is to translate and rotate one structure with respect to the other to minimize the RMSD. Coutsias, et al. presented a simple derivation, based on quaternions, for the optimal solid body transformation (rotation-translation) that minimizes the RMSD between two sets of vectors.[1] They proved that the quaternion method is equivalent to the well-known Kabsch algorithm.[2]
Contents |
The equation
where δ is the distance between N pairs of equivalent atoms (usually Cα and sometimes C,N,O,Cβ).
Normally a rigid superposition which minimizes the RMSD is performed, and this minimum is returned. Given two sets of 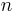 points
points 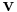 and
and 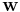 , the RMSD is defined as follows:
, the RMSD is defined as follows:
An RMSD value is expressed in length units. The most commonly used unit in structural biology is the Ångström (Å) which is equal to 10–10m.
Uses
Typically RMSD is used to make a quantitative comparison between the structure of a partially folded protein and the structure of the native state. For example, the CASP protein structure prediction competition uses RMSD as one of its assessments of how well a submitted structure matches the native state.
Also some scientists who study protein folding simulations use RMSD as a reaction coordinate to quantify where the protein is between the folded state and the unfolded state.
See also
- Root mean square deviation
- Root mean square fluctuation
- Quaternion – used to optimise RMSD calculations
- Kabsch algorithm – an algorithm used to minimize the RMSD by first finding the best rotation[2]
- GDT – a different structure comparison measure
- TM-Score – a different structure comparison measure
References
- ^ Coutsias EA, Seok C, Dill KA (2004). "Using quaternions to calculate RMSD". J Comput Chem 25 (15): 1849–1857. doi:10.1002/jcc.20110. PMID 15376254.
- ^ a b Kabsch W (1976). "A solution for the best rotation to relate two sets of vectors". Acta Crystallographica 32 (5): 922–923. doi:10.1107/S0567739476001873.
Further reading
- Shibuya T (2009). "Searching Protein 3-D Structures in Linear Time." Proc. 13th Annual International Conference on Research in Computational Molecular Biology (RECOMB 2009), LNCS 5541:1–15.
- Armougom F, Moretti S, Keduas V, Notredame C (2006). "The iRMSD: a local measure of sequence alignment accuracy using structural information". Bioinformatics 22 (14): e35–39. doi:10.1093/bioinformatics/btl218.
- Damm KL, Carlson HA (2006). "Gaussian-Weighted RMSD Superposition of Proteins: A Structural Comparison for Flexible Proteins and Predicted Protein Structures". Biophys J 90 (12): 4558–4573. doi:10.1529/biophysj.105.066654. PMC 1471868. PMID 16565070. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1471868.
- Kneller GR (2005). "Comment on 'Using quaternions to calculate RMSD' [J. Comp. Chem. 25, 1849 (2004)]". J Comput Chem 26 (15): 1660–1662. doi:10.1002/jcc.20296. PMID 16175580.
- Theobald DL (2005). "Rapid calculation of RMSDs using a quaternion-based characteristic polynomial". Acta Crystallogr A 61 (Pt 4): 478–480. doi:10.1107/S0108767305015266. PMID 15973002.
- Maiorov VN, Crippen GM (1994). "Significance of root-mean-square deviation in comparing three-dimensional structures of globular proteins". J Mol Biol 235 (2): 625–634. doi:10.1006/jmbi.1994.1017. PMID 8289285.
External links
- Molecular Distance Measures—a tutorial on how to calculate RMSD
- RMSD—another tutorial on how to calculate RMSD with example code
- Secondary Structure Matching (SSM) — a tool for protein structure comparison. Uses RMSD.
- SuperPose — a protein superposition server. Uses RMSD.
- superpose — structural alignment based on secondary structure matching. By the CCP4 project. Uses RMSD.